From Classical to Quantum – Bose-Einstein Condensation – Atomic Cooling Techniques – Timeline: Light-Matter Interaction
From Classical to Quantum
Classical Particles
Nearly all matter that we can see is made up of individual particles called atoms. Depending on the way atoms behave in its environment, different phases of matter can form. When we think about these phases, we tend to think about solids, liquids, gases. In physics, we treat these phases of matter classically – meaning that we describe the behavior of the system using classical equations like F = ma (Newton’s 2nd Law), P + (nv^2)/2 + ngz =const (Bernoulli’s Equation), and PV = nRT (Ideal Gas Law). Because our experience is only in the realm of room temperatures, this treatment is adequate. You can read more about solids, liquids, gases and plasmas at Ask Dr. Universe.
What if, however, we continuously removed energy – or heat – from a system and could actually approach absolute zero? Well, we could definitely not survive in those conditions, but atoms can! Atoms, when reaching these low temperatures start to behave differently, and instead of acting like billiard balls, atoms behave more like waves.
But how can a piece of matter act like both a particle and like a wave? Consider light.
Light
Light is something that we experience every day. When we see an object, the light is reflected from the object, projected into our eyes and is interpreted by the brain. Our brain in turn can recognize certain features of the object, like the brightness and the color of the light.
Light as a wave
We know from natural phenomena, like rainbows, that light behaves like a wave. Experiments, such as Young’s double slit experiment, also confirmed this notion. Waves have two properties, amplitude and wavelength. The amplitude tells us about the intensity and the wavelength tells us what kind – or color – of light we are seeing. Different colors and types of light have different wavelengths! So when we see objects, our eyes and our brains work together to tell us information about the color and the textures of the object. But we can only see a very narrow slice of the whole electromagnetic spectrum.
Light as a particle
We know from the photoelectric effect that light is made up of particles of pure quantized energy, known as photons. Our brains interpret the number of photons passing to our eyes in a fixed time as the brightness. If there are too many photons, we instinctively squint or close our eyes. Remember: Never look directly at the sun!
Wave-Particle Duality
This is a simple way of thinking about light being a particle and a wave at the same time. This concept is known as wave-particle duality, and it applies to more than massless particles!
All matter has both particle and wave properties! The matter wavelength is defined as Planck’s constant h over the momentum. As energy is removed from a system and it experiences lower and lower temperatures, the speed of each particle in the system is slowed. The matter wavelength is then found to be inversely proportional to the root of the temperature. So as the temperature gets smaller, the wavelength gets larger, and visa versa!
![]()
This wave-y nature of matter is an effect of quantum mechanics! As observers, we can see this wave-like nature of matter in two ways:
-
If we look on a very small, atomic scale
-
If we make things very cold.
And making things very cold, or ultracold, is what I do in my lab!
Minute Physics: Wave-Particle Duality Part 1 Part 2
Bose-Einstein Condensation
From Classical to Quantum – Bose-Einstein Condensation – Atomic Cooling Techniques – Timeline: Light-Matter Interaction
Using the quantum mechanical principle of wave-particle duality, we can now better understand what a dilute-gas Bose-Einstein condensate, or BEC, is.
When a group of atoms is made colder and colder, using techniques like laser cooling (see next page!), the matter wavelength of these atoms becomes longer and longer. Eventually the matter wavelength becomes comparable to the average distance between the atoms in the gas, which is a measurement of the density of the system, and the matter wavelengths begin to overlap. This is the critical point* for a BEC! At this point, the atoms in the gas start to populate a ground state (or low energy state) in the system!
Once bosons start to populate the ground state, the remaining atoms in the system at higher energies want to also fall into the ground state. Very quickly, the percentage of atoms that have been Bose condensed increases, ideally reaching around 100% at absolute zero. When multiple atoms occupy the same state, this is called quantum degeneracy.
In our experiments, this percentage is typically between 85-99%, depending on your system, the procedures used to cool your atoms, and the amount of heating induced by external fields. The existence of a thermal cloud is not normally an issue, as the Bose condensed atoms don’t interact very strongly with the surrounding thermal cloud in the system. Once the atoms are Bose condensed, they start to exhibit peculiar features, such as superfluidity and phase coherence, meaning that they flow without internal resistance and – just like light or a water wave – can interfere with itself!
On the next page, I explain some of the common steps and techniques used for cooling atoms to these low temperatures.
Atomic Cooling Techniques
From Classical to Quantum – Bose-Einstein Condensation – Atomic Cooling Techniques – Timeline: Light-Matter Interaction
Now, let’s focus on some cool techniques (pun intended).
Using lasers to cool atoms
You may be thinking to yourself: “What??? How can lasers possibly cool atoms? Don’t lasers heat things up, like in the movies?”
Well, it is true that lasers can heat things up! However, it really depends on what the color of the light is and what the laser is pointing at.
Atomic Fingerprints
Every single type of atom has a fingerprint. This fingerprint tells us how valence electrons – or the electrons on the outermost part of the atom – can move in an atom when they are excited. You can excite electrons using light. In the quantum world, energies can be quantized. This means that a bound electron can only exist at energies specific to the type of atom it lives in.
If you were to look at these fingerprints, you would notice that atoms located under hydrogen on the periodic table (see red boxes on periodic table) are all pretty simple. These are neutral Alkalai atoms and they all have exactly one valence electron. As you start to work your way across the periodic table, these fingerprints become very complicated because more electrons can be excited in those atoms.
Check out below to see some examples of fingerprints, or spectra, of some common elements!

Periodic Table of Elements
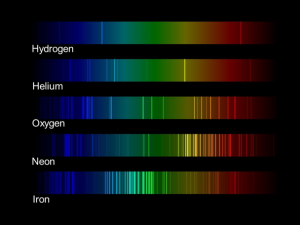
Fingerprint, or spectrum, of various common elements. Image Credit: NASA JPL, “Using light to study planets,” Classroom activity.
Now that you know some more about atoms and the way electrons can move inside an atom, we can talk about laser cooling!
Light-Matter Interaction
Light is pure energy and all light comes about from charged particles moving – specifically accelerating! When an atom is submerged in light – or a bath of photons – electrons in the atom can become excited by absorbing the energy of a photon. The electron then jumps to a higher energy state. However, electrons are like kids on a sugar high – they only have a finite amount of time that they stay THAT excited. After that, they jump back into a lower energy state, perhaps even to the same energy where they started. But to do this, an electron must re-emit energy. It does this in the form of a photon of a very specific energy. This is where those fingerprints shown above come from!
Laser cooling and the Doppler effect
In laser cooling, we use the internal structure of atoms to excite electrons to higher energies. Only now, we use the Doppler effect to excite the atoms! You see, the electrons in atoms are very particular about the type of light that they like to absorb. So, instead of shining just the right type of light onto the atoms, we shine light that has a slightly lower energy, or red-detuned.
The Doppler effect tells us that if a wave-producing object is moving towards us, the waves will be compressed, whereas an object moving away from us will have stretched waves. Compressed waves have a higher frequency and stretched waves have lower frequency. The Doppler effect we typically think of has to do with sound waves from a car horn or siren, but it also works for light – this is how astronomers know if a star is moving away or towards us!

When we tune our laser light to be slightly less energetic than what we would need to excite atoms sitting still, we make the assumption that our atoms are moving. Then, if an atom is moving towards a beam of light, the light will compress and become just the right energy to be absorbed. Otherwise, an atom moving away from the light will see the light stretched and will not be excited. In this way, we tune our light so that atoms only absorb photons from one direction!
Well, now the light has been absorbed from one direction and the electron is excited. Just like a ball at the top of a hill, the electron wants to be at lower energy, so it spontaneously re-emits a photon in a random direction, and thus changing the speed and direction of the atom’s motion. This process of absorbing and re-emitting happens over and over again, rapidly, until the atoms reach a limit of how slow – or cold – they can be. This limit is proportional to the intensity of the light on the atoms over the amount we have tuned away from where the atoms are excited when sitting still, or the detuning.
Now, our atoms are pretty cold – approximately 150 micro-Kelvin. That is, we are 0.000150 degrees above absolute zero, the limit of temperature. To put this into perspective, the distance between Pullman, WA and Boston, MA is approximately 2800 miles. If absolute zero is Pullman and room temperature is Boston, the temperature of our atoms at this point would be 2 1/4 meters away from the center of Pullman. Atoms at this temperature can be used for various types of sensors, but for our purposes they still aren’t cold enough!
Trapping atoms with magnets and lasers
Now that our atoms are really cold, we want to capture them in either a trap made of magnets or laser light!
Magnetic Trapping
A magnetic trap creates a potential well for our cooled atoms to sit in, like a bowl, where the potential is proportional to the strength of the magnetic field. However, a uniform magnetic field cannot trap the atoms. Instead, we need a magnetic field with a gradient, which simply means that the strength of the magnetic field varies over space. This can be easily created by taking two coils with opposite flowing current, depicted below.

The two copper colored windings represent two sets of wound wire, known as a coil. The direction of the current, I, in one coil is opposite to the other. The green arrows represent the direction of the magnetic field around the coils. This orientation is called anti-Helmholtz and creates a magnetic field with a gradient in the center, which is where the atoms like to collect.

A magnetic trap, similar to a bowl. The hot, high energy atoms (red) move high in the bowl, while the cold, low energy atoms (blue) like to live near the bottom of the trap.
Optical Trapping
Before, when we were initially cooling the atoms, the 6 intersecting laser beams created a trap for the atoms. The confinement there was pretty weak. For the next stages in cooling, we want the atoms to help us out and do some of the cooling by bouncing off one another, or scattering. This requires that the atoms sit in a tighter trap. Using a high intensity focused laser beam that has a larger wavelength, i.e. red detuned light, than the light used for laser cooling, we can strongly trap the atoms with light!
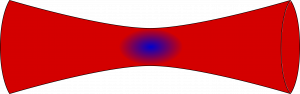
Concept of an optical trap, where the light source is focused down and the atoms sit at the center of the trap, where the potential is deepest. This is the same technique used for optical tweezers!
The force applied on the atoms by the laser is proportional to the spatially (radially) dependent intensity over the detuning of the light from the resonant transition (the light used to cool the atoms initially).
Evaporative Cooling
Now that our atoms are relatively chilly and strongly trapped, we can proceed with the second, and sometimes final, stage of cooling our atoms!
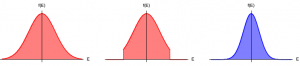
Concept of evaporative cooling process. Left: The thermal distribution of atoms prior to evaporative cooling at temperature T1. Middle: Distribution after applying an evaporative cooling method, still at temperature T1. Right: Thermal distribution after rethermalization, at temperature T2, where T2<T1.
…In a magnetic trap
If the atoms are tightly confined in a magnetic trap, we can apply radio frequencies (RF) or microwave (MW) frequencies to kick hot atoms out of the trap, allowing the remaining cold atoms to rethermalize to a colder temperature.
In addition to the atomic fingerprints mentioned previously, the internal states of an atom will separate further with the application of a magnetic field. This is called the Zeeman effect!
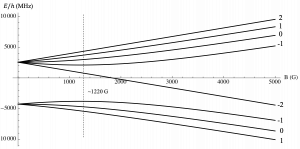
(a) Rubidium-87
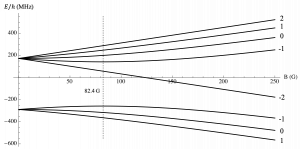
(b) Potassium-39
Explanation of above figures: Zeeman splitting of the 5s level ground state for increasing magnetic fields in (a) Rubidium-87 and (b) Potassium-39 atoms. The lower lines are the F = 1 hyperfine manifold (m_F = -1, 0, +1) and the upper lines are the F = 2 hyperfine manifold (m_F = -2, -1, 0, +1, +2). Lines with a downward slope are non-magnetically trappable. The vertical dashed line indicated the magnetic field at which the (F, m_F) = (1, -1) state is no longer magnetically trappable.
If the field is constant across the system, the atoms will see the same magnetic field all have the same state separation, or Zeeman splitting. However, if the atoms are sitting in a magnetic trap, the state separation changes as a function of position! So hot atoms, moving with higher energy, are on average farther from the center of the trap and therefore at higher magnetic fields!

Zeeman splitting in a magnetic trap with magnetic gradient of 180 G/cm. We can apply RF frequencies to excite atoms from the m_F = -1 state to the m_F = 0 and then m_F = +1 states.
At this point, we can eliminate these hot atoms at high fields by applying RF or MW frequencies to the system. This causes hot atoms in the magnetically trappable m_F = -1 state to transition to the slightly non-trappable m_F = 0 state. The atoms then start to roll out of the trap, and are then resonantly excited to the m_F = +1 state, which is highly non-trappable and the atoms are ejected out of the system.
The remaining atoms in the trap can then scatter off of one another, coming to a new thermal equilibrium. This process is known as rethermalization and must be done slowly. If done too quickly, the atoms cannot go through this process and you do not efficiently cool your atoms! In our apparatus at WSU, this process takes 4 seconds.
Now, if you have a special type of magnetic trap where there is no region of the trap where the magnetic field goes to zero, such as an Ioffe-Pritchard type trap or a Time-averaged Orbiting Potential (TOP) trap, you can perform this evaporative cooling technique until all of your atoms Bose condense into a single energy state. The TOP trap was first used by the Cornell JILA group and successfully cooled atoms to Bose condensation for the first time in 1995. To hear more about this discovery, I recommend taking the time to watch or read Eric Cornell’s Nobel lecture.
If you trap has a magnetic zero, your atoms will become unhappy when they are cooled past a specific point in your trap and undergo dynamics where they cannot maintain the direction of their spin in the trap. These are called Majorana losses and cause rapid loss of atoms. One way to overcome these losses is to “optically plug” the center of the magnetic trap. By using a wavelength of light that is repulsive to the atoms, i.e. less than the resonant frequency, the atoms are not able to pass through regions of the field that are near or at zero magnetic field. This is the technique used by the MIT team to Bose condensed their sodium atoms in 1995 (see page 129 of Ketterle’s Noble lecture for a diagram of the experimental setup).
If not using a special magnetic trap, or an optical plug, you must load your atoms into a strong optical trap to eliminate any issues having to do with losses due to magnetic fields. This is a technique used in my old lab at WSU.
Atoms can also be trapped with lasers! This uses the concept of optical tweezers where a tightly focused laser beam can hold, move, and manipulate atoms. In cold atom applications, an optical trap can consist of a single optical tweezer, forming a cigar shaped cloud, or can consist of multiple tweezers that intersect at the foci. This changes the dimensionality of the potential where the atoms live.
Once atoms are loaded into the optical tweezer trap, the intensity of the laser is reduced. The same underlying concept applies here in regards to evaporative cooling in a magnetic trap: kick out the hot atoms and let the remaining atoms rethermalize at new, lower temperatures. If you imagine the optical potential that holds the atoms like a canvas bowl, reducing the intensity of the laser is equivalent to peeling down the sides of the bowl. The atoms sitting at the top of the bowl flow out and the remaining atoms are left to get cooler.
This method of evaporative cooling is a much more rapid method of cooling. This has to do with the tightness of the trap that the atoms live. A tighter trap means that the atoms will collide more often, leading to faster rethermalization. It is difficult to get magnetic traps tight enough to do this quickly, with exception to chip traps. Optical traps are thus the preferred method for performing evaporative cooling down to condensation. The only caveat is that the atoms need to already be pretty cold (~10 microK) to be able to be trapped with a reasonable amount of laser intensity!
Condensation!
At the end of the evaporation cooling process, something amazing happens: Atoms undergo a phase transition from being a cold thermal gas to being an ultracold degenerate Bose condensed gas. The Bose condensed gas, is a quantum mechanical object where all of the atoms live in the ground state of the system, aka they are degenerate. Once atoms start to condense, the rest of the atoms rapidly undergo this transition due to Bose enhancement. Essentially, lonely atoms in higher excited states see more atoms in the ground state and want to go be degenerates with them.* Eventually, the majority of atoms are condensed in the ground state and are known as a dilute-gas Bose-Einstein condensate, or a BEC.
Once our atoms are Bose condensed, we can use a number of tools found in our atomic toolbox to manipulate and probe the quantum nature of the system and create analog systems to perform quantum simulations!
Timeline: Light-Matter Interaction
From Classical to Quantum – Bose-Einstein Condensation – Atomic Cooling Techniques – Timeline: Light-Matter Interaction
-
1577: Astronomer Tycho Brahe observes the Great Comet of 1577 and discovers a comet’s coma faces away from the sun
-
1619: Johannes Kepler develops ideas to describe why a comet’s tail points away from the sun
-
1870s: James Clerk Maxwell theorizes that light could apply pressure onto a system
-
1905: Einstein develops his theory for the photoelectric effect
-
1921: Einstein wins the Nobel Prize for the photoelectric effect
-
1922-1923: Experiments by Compton and Wilson use cloud chambers to observe the recoil momentum in electrons via X-rays
-
1927: Compton and Wilson win Nobel Prize for the “Compton effect”
-
1933: Frisch demonstrates that sodium atoms can also be scattered by radiation generated by a sodium lamp
-
1960: Theodore Maiman builds the first laser based on theoretical work by Charles Townes and Arthur Schawlow.
-
1964: Charles Townes shares the Nobel prize with Nicolay Basov and Aleksandr Prokhorov for the development of the maser and the laser
-
1975: Two separate teams, Hänsch & Schawlow and Wineland & Dehmelt, propose cooling atoms (ions) using laser light
-
1978: Arthur Ashkin describes how lasers can be used to optically trap and cool atoms, laying groundwork for creating the first optical tweezers
-
1982: William Phillips leads a team to create the first laser-cooled neutral atoms using a Zeeman slower
-
1985: Stephen Chu and Claude Cohen-Tannoudji in separate experiments show the cooling of neutral atoms in an optical molasses
-
1995: Teams at CU Boulder and MIT separately observe Bose condensation of neutral atoms.
-
1997: Phillips, Chu and Cohen-Tannoudji share the Nobel Prize for laser cooling neutral atoms
-
2001: Eric Cornell, Carl Wieman and Wolfgang Ketterle share Nobel Prize for first creating Bose-Einstein condensates
-
2018: Ashkin shares the Nobel Prize with Gérard Mourou and Donna Strickland for inventions in the field of laser physics, with Ashkin winning for the development of optical tweezers
